

The Layden Lab studies neural development in the sea anemone Nematostella vectensis. Nematostella is an emerging system that is important, because it is a robust model for studying mechanisms that regulate neurogenesis during development and regeneration. Understanding the similarities and differences in how developmental programs are redeployed during regeneration will provide critical clues necessary to better design regenerative therapies for biomedical applications.
Nematostella is also a cnidarian animal that possesses a nerve net rather than a central nervous system. Cnidarians (jellyfish, corals, sea anemones, hydras) are a phylogenetically pivotal group of animals, because they are the closest relatives to the bilaterians (insects, annelids, mollusks, sea urchin, vertebrates). One characteristic of most bilaterians is the presence of a centralized nervous system. By investigating neurogenesis in cnidarians we can infer the ancestral mechanisms that gave rise to and perhaps evolutionary origin of the bilaterian central nervous system(s).

Click here to learn about Dr. Layden's research in the Fall 2015 issue of Acumen, a publication of Lehigh University's College of Arts and Sciences.
Characterizing the role of the neurogenic transcription factor NvashA during development and regeneration
Characterizing transgenic lines
NvashA regulates formation of a subset of the Nematostella nervous system during embyrogenesis. We will continue to disrupt NvashAfunction and assay neural phenotypes at larval and juvenile polyp stages. In conjunction with this we are improving approaches to disrupt NvashA function during regeneration. The goal is to assay whether NvashA regulates formation of the same neurons that it does during development, regulates neural development using the exact same molecular program, and/or regulates distinct neuron sub-types that are not regulated by NvashA during development.
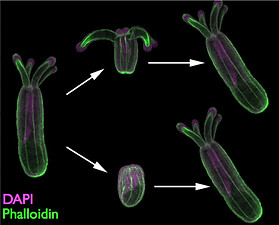
As part of our work investigating NvashA we have generated a number of transgenic lines. We are in the process of characterizing the types of neurons described by the transgenic lines, mapping the projections from each neuronal type, and describing re-development of neurons labeled by transgenic reporters during regeneration.


Investigating the evolution of Notch signaling as a regulator of neural development.
We recently reported about the role of Notch signaling as a regulator of neural differentiation, and in particular of its role regulating NvashA-dependent neurogenesis. We found some key differences between Notch signaling in Nematostella and in bilaterians. Current work aims to better understand the molecular mechanism by which Notch regulates neurogenesis in cnidarians in order to better understand the evolution of Notch signaling and neural differentiation.

